A recent survey showed that almost two-thirds of adults are unaware of the signs of bladder cancer with 10 having never even heard of the disease. RocheGenentech Highlights in cancer immunotherapy 27 Urothelial Bladder Cancer.

Roche Roche Delivers Good Sales Growth In The First Nine Months Of 2016
50 of patients who undergo radical surgery for invasive disease will most likely suffer a relapse.

Roche bladder cancer immunotherapy. In a single-arm 310-patient trial nearly 15 percent of participants saw their tumors shrink. 2 Common symptoms include 3. Roche are leading in cancer immunotherapy through innovative research including immune profiling with the goal to develop treatment tailored to individuals.
Tecentriq failed to improve survival compared to chemotherapy in second-line bladder cancer patients. Cancer Immunotherapy Fallout From Roches Bladder Cancer Failure. One of the groups of people most at risk of bladder cancer are older men 5 and this proved true for Dave from Torquay UK.
More advanced cancers may require removal of the entire bladder cystectomy. ZURICH Reuters Roche is withdrawing its cancer immunotherapy Tecentriq from US. Basel 19 May 2016.
Bladder cancer In 2016 Roche won the first approval for its immunotherapy Tecentriq in urothelial cancer which affects the lining of the bladder and other urinary tissue. Continued research into tumour biology and the introduction of cancer immunotherapies has brought new hope and a new option for certain people with bladder cancer after 30 years without advances. Bladder Cancer Immunotherapy Get FDA Nod.
The hope is that the combination of earlier diagnosis and recent treatment advances will help improve survival rates for people with bladder cancer. 90 of treatment for bladder cancer is surgery combined with other therapies including chemotherapy radiation therapy and immunotherapy. Enfortumab vedotin is infused into a vein IV once a week for 3 weeks with one week off.
FDA grants Roches Cancer Immunotherapy Atezolizumab priority review for advanced bladder cancer. Roche could not confirm. The test may help identify patients most likely to respond to Roches new metastatic urothelial cancer drug Tecentriq.
Learn about TECENTRIQ atezolizumab a cancer Immunotherapy treatment for Metastatic Non-Small Cell Lung Cancer mNSCLC Extensive-Stage Small Cell Lung Cancer ES-SCLC Liver cancer HCC Metastatic Triple-Negative Breast Cancer mTNBC Advanced Melanoma Advanced Bladder Cancer mUC TECENTRIQ atezolizumab. 1 The most common type of bladder cancer is urothelial cancer also known as transitional cell carcinoma which accounts for 9 out of 10 bladder cancers. The chemo enters the cancer cells and kills them.
Currently one of the main treatments for non-muscle-invasive bladder cancer is an immune-based therapy called BCG bacillus Calmette-Gurin. Food and Drug Administration FDA has accepted the companys Biologics License Application BLA and granted Priority Review for atezolizumab anti-PDL1. AstraZenecas Imfinzi and Mercks Keytruda quickly followed a year later.
RHHBY today announced that the US. FDA Approves Roche Immunotherapy for Bladder Cancer Roches Genentech developed Tecentriq also known as atezolizumab which shrank tumors in patients in a clinical study The FDA has approved. The Roche PCI research and development programme comprises more than 20 investigational candidates ten of which are in clinical trials.
Bladder cancer is the 6th most commonly occurring cancer in men and the 17th most commonly occurring cancer in women globally. First and only anti-PD-L1 cancer immunotherapy approved by the FDA. Patients are given a weakened form of the bacterium Mycobacterium bovis in a solution to stimulate the immune system against cancer.
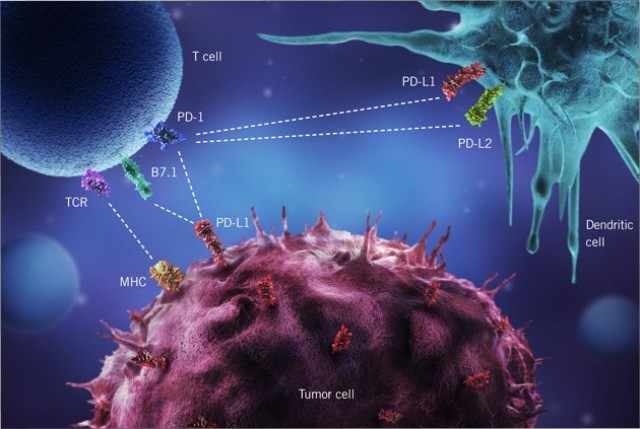
Tecentriq is the fourth medicine of a new class called checkpoint inhibitors that work by unleashing the bodys immune system to attack tumors. This is the first PD-L1 inhibitor approved for bladder cancer. Anti-OX40 anti-CD40 INO-5150 aPDL1 Gazyva aPDL1 immune modulators Phase 3 bladder Undisclosed cancer type Cancer Immunotherapy ASCO 2014.
Use in treating prior-platinum treated metastatic urothelial carcinoma or bladder cancer the Swiss drugmaker said Monday after follow-up trials failed to meet goals. 1 Yet bladder cancer is one of the top 10 most common cancers in the world. Bladder cancer and proof of concept for anti-CSF-1R 9 ongoing trials 6 new clinical trials planned in 2014.
FDA grants Roches cancer immunotherapy Tecentriq atezolizumab accelerated approval for people with a specific type of advanced bladder cancer. PCI is an essential component of how Roche delivers on the broader commitment to personalised healthcare. To learn more about the Roche approach to cancer immunotherapy please follow this link.
Bladder cancer can be categorised as non-muscle invasive muscle invasive or. Roche is betting a new type of cancer immunotherapy could help to improve on the benefit offered by the first generation of immune-boosting drugs. This drug may be used to treat people with advanced bladder cancer who have already been treated with a platinum chemo drug such as cisplatin and immunotherapy specifically a PD-1 or PD-L1 inhibitor.
First FDA-approved treatment for people with a specific type of bladder cancer in more than 30 years. Basel 15 March 2016.
Roche Gets Fda Priority Review For Atezolizumab European Biotechnology
Roche To File Tecentriq In Triple Negative Breast Cancer
Https Www Roche Com Dam Jcr 5d791319 Ac90 4814 9c8b 40c6327d533e De Irp170727 Annex Pdf
Roche Signs 310mm Early Stage Deal With For Mrna Vaccines Cancer Biology
Roche Roche To Present New Positive Data From Its Broad Cancer Immunotherapy Programme And Across A Wide Range Of Cancers At The European Society For Medical Oncology Esmo 2018 Congress

Roche Ramps Up Immunotherapy R D European Biotechnology

Bladder Cancer Therapeutics Market Current Scenario 2020 Global Size Trends Growth By Top Leaders Dynamic Business Opportunity And Challenges From Up To 2026 Medgadget
Roche S Tecentriq Fails Trial In Form Of Urothelial Cancer Reuters
Asco Roche Looks For Bladder Cancer Clues In Tecentriq S Post Surgery Flop Fiercepharma
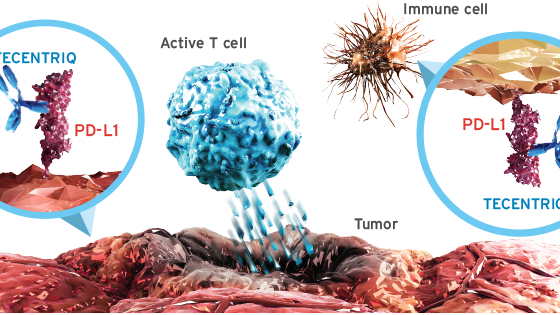
Roche S Anti Pd L1 Fails In Bladder Cancer European Biotechnology

Roche Fda Grants Roche S Tecentriq Atezolizumab Accelerated Approval As Initial Treatment For Certain People With Advanced Bladder Cancer

Cancer Immunotherapy In Hcc The Combination Revolution Esmo
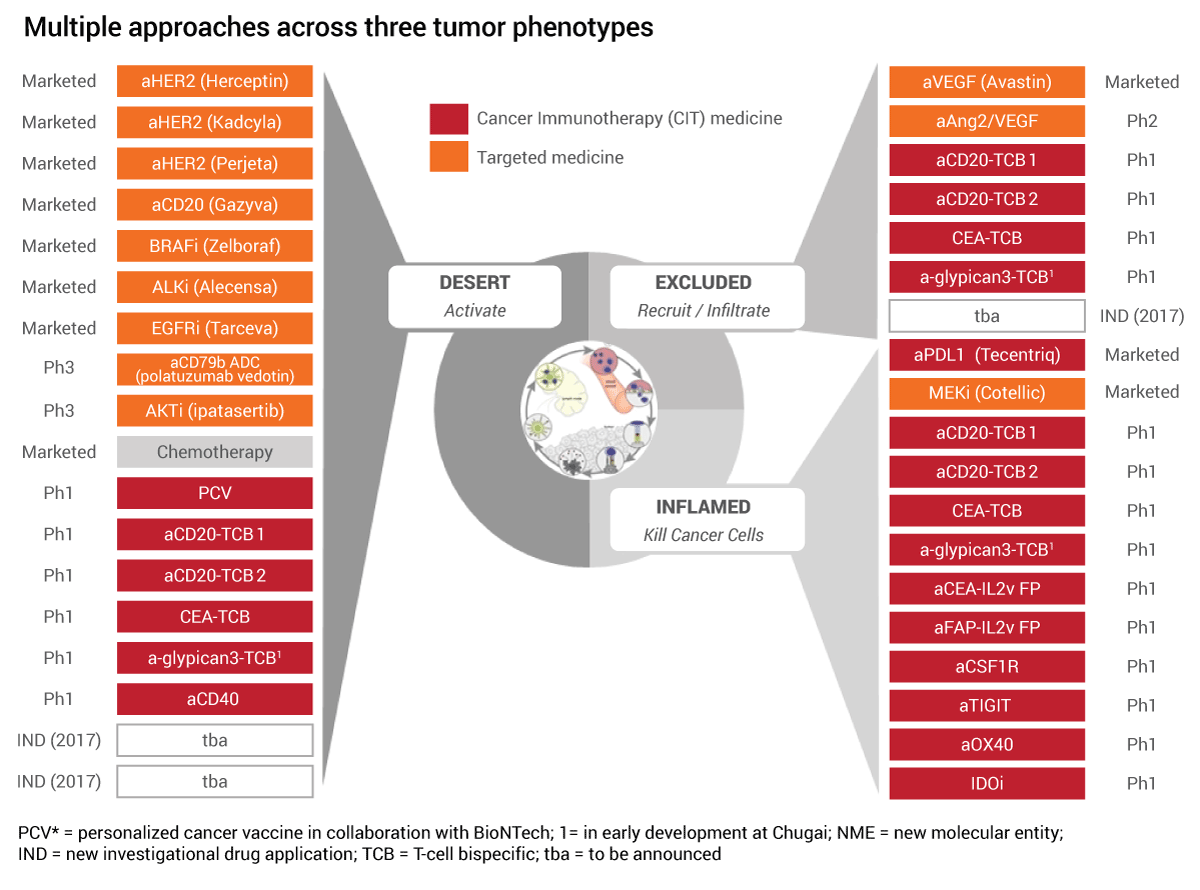
Roche Pushes The Boundaries Of Cancer Immunotherapy Scrip
Positive First Line Phase 3 Results For Tecentriq Pmlive

Uk Mhra Recommends Roche S New Immunotherapy Atezolizumab For Bladder Cancer