Skin cancer melanoma or Merkel cell carcinoma. Keytruda was granted accelerated approval as first-line therapy for patients who cannot receive standard of care chemotherapy containing Platinol cisplatin.
Cancer Immunotherapy Drugs Like Keytruda And Opdivo Hold Hope For Some But There S Still A Way To Go
KEYTRUDA may be used when your bladder or urinary tract cancer.

Keytruda treatment for bladder cancer. Only your doctor treating your cancer can answer how well Keytruda might work for you. In general Keytruda is much easier that cisplatin and other chemo. Head and neck cancer.
KEYTRUDA may be used when your bladder or urinary tract cancer. KEYTRUDA is a prescription medicine used to treat a kind of bladder and urinary tract cancer called urothelial carcinoma. Meanwhile it is being evaluated as monotherapy and in combination with other anti-cancer.
Health 8 days ago KEYTRUDA is a prescription medicine used to treat a kind of bladder and urinary tract cancer called urothelial carcinoma. Has spread or cannot be removed by surgery advanced urothelial cancer and. Cancer of the cervix or uterus.
Has spread or cannot be removed by surgery advanced urothelial cancer and. Keytruda has been approved in the US. It might be.
Keytruda was approved for use in the US in 2014 and it was only approved as a first-line treatment for metastatic bladder cancer in 2019. Pembrolizumab Keytruda is an Immunotherapy Regimen for Bladder Cancer How does pembrolizumab work. Food and Drug Administration has approved Mercks anti-PD1 therapy Keytruda pembrolizumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma or bladder cancer.
KEYTRUDA is a prescription medicine used to treat a kind of bladder cancer called urothelial carcinoma. Cancer treatment is always individual and what you may experience may be different from someone else. In March this year Merck reported that Keytruda improved progression-free survival PFS in relapsed or refractory classical Hodgkin lymphoma cHL patients involved in the Phase III KEYNOTE-204 clinical trial.
Nadofaragene firadenovec which uses an adenovirus vector to deliver the gene interferon alfa-2b. FDA approves Keytruda for bladder cancer subset The FDA approved pembrolizumab for treatment of certain patients with bacillus Calmette-Gurin. KEYTRUDA may be used when your bladder or urinary tract cancer.
Early data from two clinical trials 1 show reduced survival with Keytruda pembrolizumab and Tecentriq atezolizumab when used as first-line treatments for urothelial cancer cancer of the bladder and urinary tract in patients with low levels of a protein called PD-L1. Its important that you are near a teaching hospital that will be able to diagnose and treat you if you get immunotherapy toxicity from Keytruda. Primary mediastinal large B-cell lymphoma.
KEYTRUDA is a prescription medicine used to treat a kind of bladder and urinary tract cancer called urothelial carcinoma. If your husbands pathology mutations suggest Keytruda it. This is commonly called immunotherapy.
Keytruda had its own victory in that department having recently become the first PD-1L1 drug to win an FDA nod to treat non-muscle invasive bladder cancer thats. I am receiving Keytruda for bladder cancer. Data show lower survival in some patients with low levels of cancer protein PD-L1.
And in the EU to treat different types of cancer including lung bladder stomach esophageal and liver cancer lymphoma melanoma and cancers with specific genetic features that failed prior therapies. Most of the side effects are less dangerous than dying from bladder cancer. Keytruda Improves Survival of Advanced Bladder Cancer.
When my oncologist said this would be the treatment route we were going to take I admit I had no idea what immunotherapy was. Keytruda pembrolizumab is a prescription medicine used to treat many different types of cancers including solid tumors and blood cancers. Has spread or cannot be removed by surgery advanced urothelial cancer and.
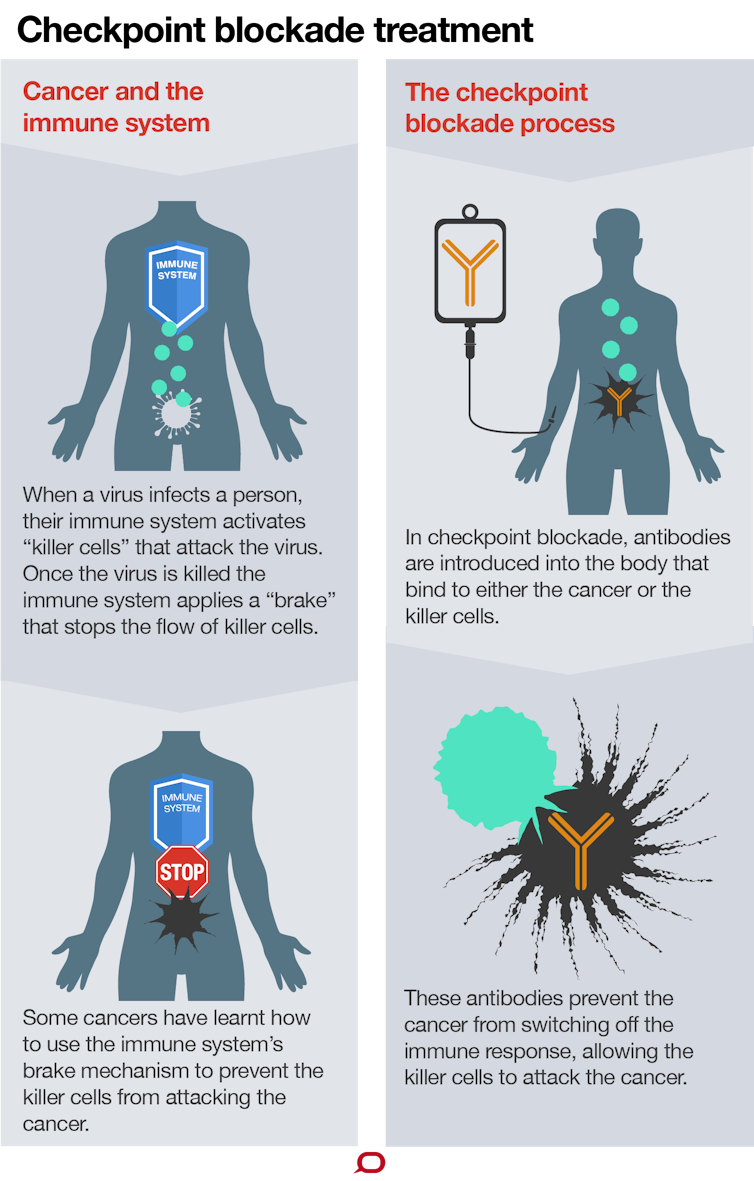
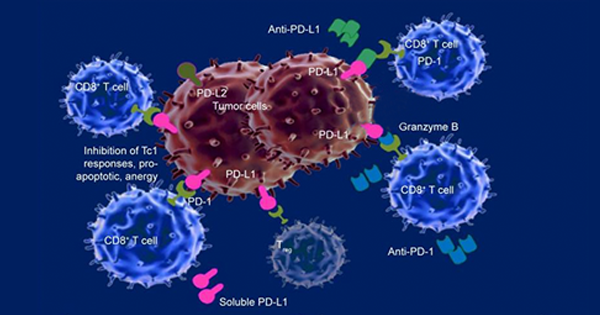
Pembrolizumab is designed to block tumor cell signals that suppress the immune system and stop the immune system from attacking bladder cancer cells. Merck is further assessing Keytruda as a monotherapy and combination therapy across several disease settings for bladder cancer. Keytruda is meant to be given at a fixed dose every three weeks.
FDA approves pembrolizumab for BCG-unresponsive high-risk non-muscle invasive bladder cancer. Keytruda is used alone or in combination with other medicines to treat certain types of cancer such as. On January 8 2020 the Food and Drug Administration approved pembrolizumab KEYTRUDA Merck Co.
Immunotherapy for Advanced Urothelial Bladder Cancer. KEYTRUDA may be used when your cancer has not spread to nearby tissue in the bladder but is at high-risk for spreading high-risk non-muscle invasive bladder cancer NMIBC and when your tumor is a type called carcinoma in situ CIS and you have tried treatment with Bacillus Calmette. Cancer of the kidney bladder and urinary tract.
Patients with recurrent urothelial cancer lived longer when they received Keytruda pembrolizumab compared to chemotherapy as second-line treatment according to long-term follow of a clinical trial presented at the Genitourinary Cancer Symposium. My nurse educator actually had the best analogy to help me understand. For bladder cancer it is approved for three indications across different types and disease settings.
Checkpoint Inhibitor Use Changed For Bladder Cancer National Cancer Institute
Melanoma Therapies With Keytruda Pembrolizumab Advanced Melanoma Adjuvant Therapy Patients

Pin On Fda Approvals New Cancer Drugs

Pembrolizumab Monotherapy For The Treatment Of High Risk Non Muscle Invasive Bladder Cancer Unresponsive To Bcg Keynote 057 An Open Label Single Arm Multicentre Phase 2 Study The Lancet Oncology

Clinical Trials In Previously Treated Advanced Urothelial Bladder Cancer Patients
:max_bytes(150000):strip_icc()/pseudoprogression-with-cancer-treatment-4692751_FINAL-63b9108eb2ae4328a1e5fb065f3673c2.png)
Pseudoprogression With Immunotherapy Treatment For Cancer

Immunotherapy For High Risk Non Muscle Invasive Bladder Cancer Patients
Bladder Cancer Treatment Bladder Cancer Pictures Signs Symptoms To Better Understand Diagnosis Cleveland Oh University Hospitals

Immunotherapy For Advanced Urothelial Bladder Cancer Patients

Treatment Of Stage Iv Bladder Cancer Cancerconnect

Two Fda Wins For Immunotherapy In Bladder Cancer Medpage Today
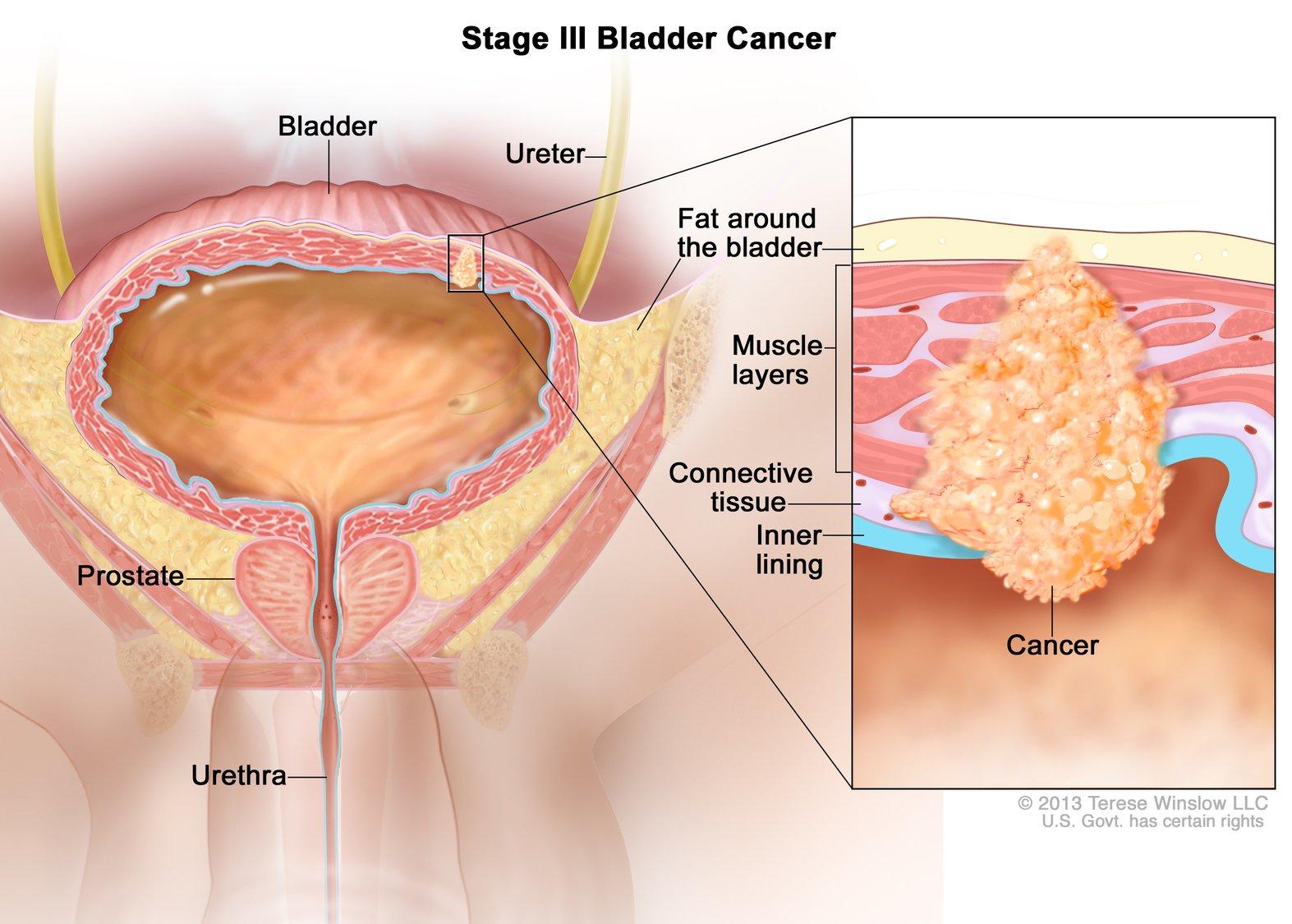
Fda Approves Nivolumab For Bladder Cancer National Cancer Institute
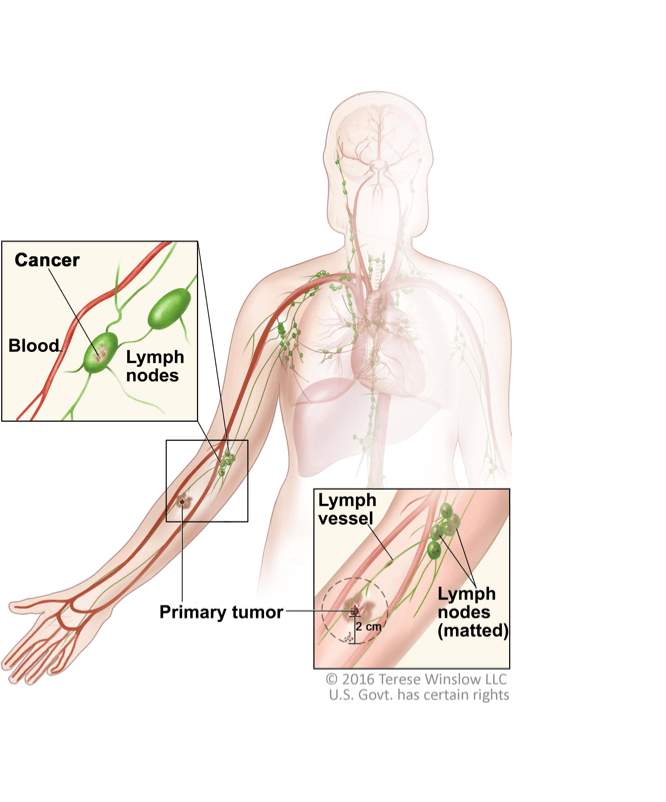
Melanoma Therapies With Keytruda Pembrolizumab Advanced Melanoma Adjuvant Therapy Patients
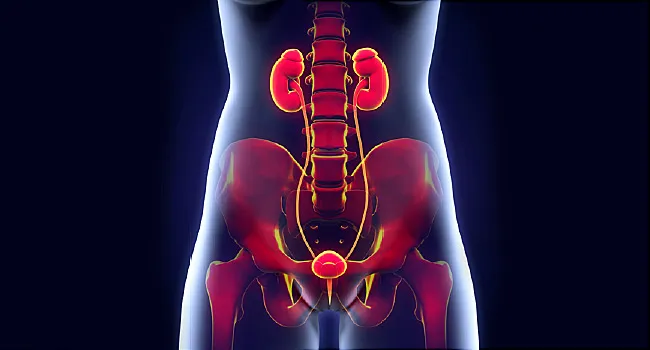
Immunotherapy For Bladder Cancer
Merck S Keytruda Fails To Hit Mark In First Line Bladder Cancer Treatment Trial Biospace

Treatment Talk Immunotherapy For Treating Bladder Cancer Bladder Cancer Advocacy Network

Immunotherapy Just After Chemo May Allow Some Patients With Bladder Cancer To Maintain Health Benefits



